Published April 11, 2020. Updated July 15, 2020. Open access. | Purchase book ❯ |
Fer-de-Lance (Bothrops asper)
Reptiles of Ecuador | Serpentes | Viperidae | Bothrops asper
English common names: Fer-de-Lance, Central American Lancehead, Terciopelo, Yellow-Jaw Tommygoff.
Spanish common names: Equis de la costa, equis, rabihueso (Ecuador); barba amarilla, cuatronarices (Colombia); terciopelo, barba amarilla, nauyaca (Central America).
Recognition: ♂♂ 220 cmMaximum distance from the snout to the tip of the tail. ♀♀ 250.1 cmMaximum distance from the snout to the tip of the tail.. In Ecuador, the Central American Lancehead (Bothrops asper) may be identified by having a triangular-shaped head with a snout that is not upturned, heat-sensing pits between the eyes and nostrils, and a dorsal pattern of 14–28 pale X-shaped markings on a brownish dorsum.1,2 Newborn lanceheads have brightly-colored tail-tips (yellowish in males and pink/whitish in females).3 In Ecuador, the most similar species that may be found living alongside B. asper are B. punctatus and B. osbornei, which are identified by their dark brown trapezoidal blotches or spots arranged in such a way that they form squares.4 The Ecuadorian Toadhead (Bothrocophias campbelli) has an upturned snout and comparatively much smaller eyes.5 The hognosed-pitvipers (genus Porthidium) have an upturned snout and a stout body.

Figure 1: Individuals of Bothrops asper from FCAT Reserve, Esmeraldas province, Ecuador (); Cerro de Hayas, Guayas province, Ecuador (); Morromico, Chocó department, Colombia (); Cerro Blanco Protected Forest, Guayas province, Ecuador (); Las Balsas Reserve, Santa Elena province, Ecuador (); Buenaventura Reserve, El Oro province, Ecuador (); and Canandé Reserve, Esmeraldas province, Ecuador (). j=juvenile.
Natural history: Generally frequentRecorded weekly in densities below five individuals per locality. to extremely commonLikely to be seen every day, usually in large numbers., especially in areas where prey is abundant, such as swamps,6 streams, and near mammal burrows,7 but uncommon in cold, pristine cloud forests.5 Bothrops asper inhabits old-growth to heavily disturbed evergreen to deciduous lowland and foothill forests, savannas, plantations (cacao, coffee, banana, and African palm), pastures, rural gardens, and even human dwellings.8–10 It also occurs, but is less abundant, in drier areas such as dry shrublands.7,10 During dry periods, individuals actively seek wetter spots near creeks and streams.2
Throughout the day, Central American Lanceheads typically remain coiled in the forest floor11 (usually close to logs, large trees, or clusters of dense vegetation)2 or sheltered in holes, below logs, or among roots,2,9 but others remain out in the open, basking in direct sunlight12,13 or moving at ground level.9,14 Within about an hour of sunset, most individuals emerge from their hideouts and move (usually less than 10 m) to their nocturnal ambush sites;12 others may remain hidden for 3–6 days, especially after a meal.6 During nights when the ambient temperature is ideal (21–31°C),15 the vipers spend an average of 37 minutes moving, but they move less during cold nights.12 Although mostly sedentary, individuals can occasionally move up to 1.2 km in two nights.2 Individuals of Bothrops asper usually dwell on soil or leaf-litter, but also sit-and-wait on the surface of slow-moving bodies of water,9 swim across rivers,12 or forage on arboreal vegetation up to 7 m above the ground.16,17 Overall, there is a tendency for juveniles to be more arboreal than adults.18,19 The home range size of the Fer-de-Lance is 0.59–13.81 ha (about the size of 1–19 soccer fields).12
Central American Lanceheads are ambush predators.12 They wait for prey to pass by. They can “bite and hold” their prey or “bite and release,” subsequently following the scent trail of the envenomated prey.12 As juveniles, they attract prey by means of moving their brightly colored tail as a lure.20 Terciopelos are opportunistic predators; they feed on almost any animal that is 3–75% of their body mass.2,21 Their diet includes primarily (69%) mammals (mostly rodents, but also rabbits, skunks, opossums, and even porcupines), but also amphibians (mostly frogs such as Leptodactylus labrosus, L. rhodomerus, Lithobates vaillanti, Pristimantis achatinus, Rhinella horribilis, and Smilisca phaeota, but also caecilians)9,22, lizards (whiptails, anoles, the microteiid Ptychoglossus gorgonae,1 and the worm lizard Amphisbaena alba),23 snakes (including Dipsas andiana,9 and members of their same species), birds, invertebrates (mainly centipedes,24,25 but also beetles, flies, hemipterans, ants, grasshoppers, and crayfish), fish,23 and carrion.2,25–28 When consumed, some toxic frogs cause the vipers to be sluggish and incapable of moving for nearly an hour.29 The diet of the Fer-de-Lance seems to shift from being based primarily on ectothermic (“cold-blooded”) prey as juveniles to based mostly on endothermic (“warm-blooded”) animals as adults.30,31 Individuals obtain water from their prey, from dew-laden surfaces, and bodies of water.2
Terciopelos rely on their camouflage as a primary defense mechanism.2 When threatened, some snakes flee, others give a “warning” by wiggling their tail against the leaf-litter, and some just readily attack.2,5 Predators of Bothrops asper include snakes (such as Clelia clelia, C. equatoriana, and Drymarchon melanurus),2,9 mammals (such as peccaries, skunks, coatis, and raccoons),1,2 falcons, hawks, chickens, crabs, and spiders (particularly tarantulas).2,9 There are records of adult Terciopelos being attacked and severely injured by monkeys.32 The Fer-de-Lance is parasitized by ticks, parasitic worms, and protozoans.2,33,34
The Fer-de-Lance is a venomous species (LD50The median lethal dose (LD50) is a measure of venom strength. It is the minium dosage of venom that will lead to the deaths of 50% of the tested population. 1.9–11.2 mg/kg)35,36 in which the venom of juveniles is more toxic, hemorrhagic, and kills more quickly than that of adults.37–40 In humans, the venom typically causes intense pain, swelling, bruising, bleeding, blistering, defibrination (depletion of the blood’s coagulation factors), nausea and vomiting, numbness, impaired consciousness, fever, and necrosis (death of tissues and cells).41–45 In pregnant women, it may cause fetal death.46 In poorly managed or untreated cases, it can cause amputations, permanent complications and disabilities (6% of cases), and even death (in 5–7% of cases).47–49 The prognosis is usually bad for victims that reach a hospital over six hours after the bite and for those that use traditional medicine, especially if they were bitten by a snake longer than one meter in total length.45,50 Critically envenomated victims die from intracranial hemorrhage, acute renal failure, blood poisoning, or hemorrhagic shock.50–52 However, some bites to humans involve no envenomation at all (“dry bites”).11 Bothrops asper causes 44.5–100% of snakebites throughout its range,47,53,54 probably because snakes of this species are perfectly camouflaged, abundant in agricultural areas,11,49 have a high venom yield (up to 1,530 mg or 5–6 cc of venom per bite)49,55 and toxicity, and have an aggressive self-defense behavior.30,44
“All the information which I have obtained concerning this reptile, wherever it is known, concurs in respect to the frightful effects of its bite. In a few hours the strongest man, in the best of health, becomes a corpse. The excitement of the nervous systems at first induced is followed by complete prostration; blood flows from every pore and life ebbs away with frightful rapidity. The Indians insist that the nauhyaca does not confine itself to biting when assaulted, but that it boldly attacks pedestrians, and even precipitates itself into boats coasting along the banks of a river. I will not endorse this statement, which seems to be at variance with the usual habits of serpents.”
Arthur Morelet, French naturalist, 1871.1
Fortunately, the antivenom available in Ecuador can, to a degree, neutralize the venom of Bothrops asper.57 However, the venom’s toxic and enzymatic activities differ drastically between populations35,58 and across age categories.38,39,59, For example, the protein similarities between the venom of two populations of Fer-de-Lance in Costa Rica may only be around 52%.58 Although serum therapy (antivenom) is the only recommended approach against a bite by a Terciopelo, extracts of some plants used by traditional “healers” may help alleviate and even neutralize the swelling and depletion of the blood’s coagulation factors caused by the envenomation.60
What to do if you are bitten by a Fer-de-Lance?
|
Males of Bothrops asper become sexually mature when they reach ~99.5 cm in total length; females at ~111.3 cm, or in little over 3 years,62 although females may attain a length of >2 m and a weight of 6 kg in just 2.5 years.63 The breeding season of some Fer-de-Lance populations coincides with the rainy season.2 Females are capable of delaying fertilization by storing sperm for years.2 After a gestation period of 6–8 months, females “give birth” (the eggs hatch within the mother) to 2–102 young1,49 that are 21.5–37.1 cm in total length.50,62 Females usually produce only one litter per year if environmental conditions are favorable.62 In captivity, individuals can live up to 21 years.2
Conservation: Least Concern Believed to be safe from extinction given current circumstances..64 Bothrops asper is listed in this category because the species is widely distributed, frequently encountered throughout its range, tolerates and even thrives in human-modified environments, and is considered to be facing no major immediate extinction threats.2 However, the Fer-de-Lance generally cannot survive in areas without vegetation cover.2 The substitution of traditional crops for mechanized agriculture is causing the species to be less frequently encountered or absent altogether in some areas.2,9 Other threats to the species include direct killing (terciopelos are usually killed on sight by humans alleging precautionary reasons),2,9,14 traffic mortality, and the decline in the abundance of prey. In a rainforest locality in Panamá, the occurrence rates of B. asper have decreased to cero in the period from 1997 to 2012, probably as a result of the collapse of amphibian populations.65 Still, given the Terciopelo’s formidable capacity to adapt to new environments, it is unlikely that it will become extinct, at least not in the near term future.2
Distribution: Bothrops asper is native to the Neotropical lowlands and adjacent mountainous areas from México to northwestern Perú. In Ecuador, it occurs at elevations between 0 and 1642 m.
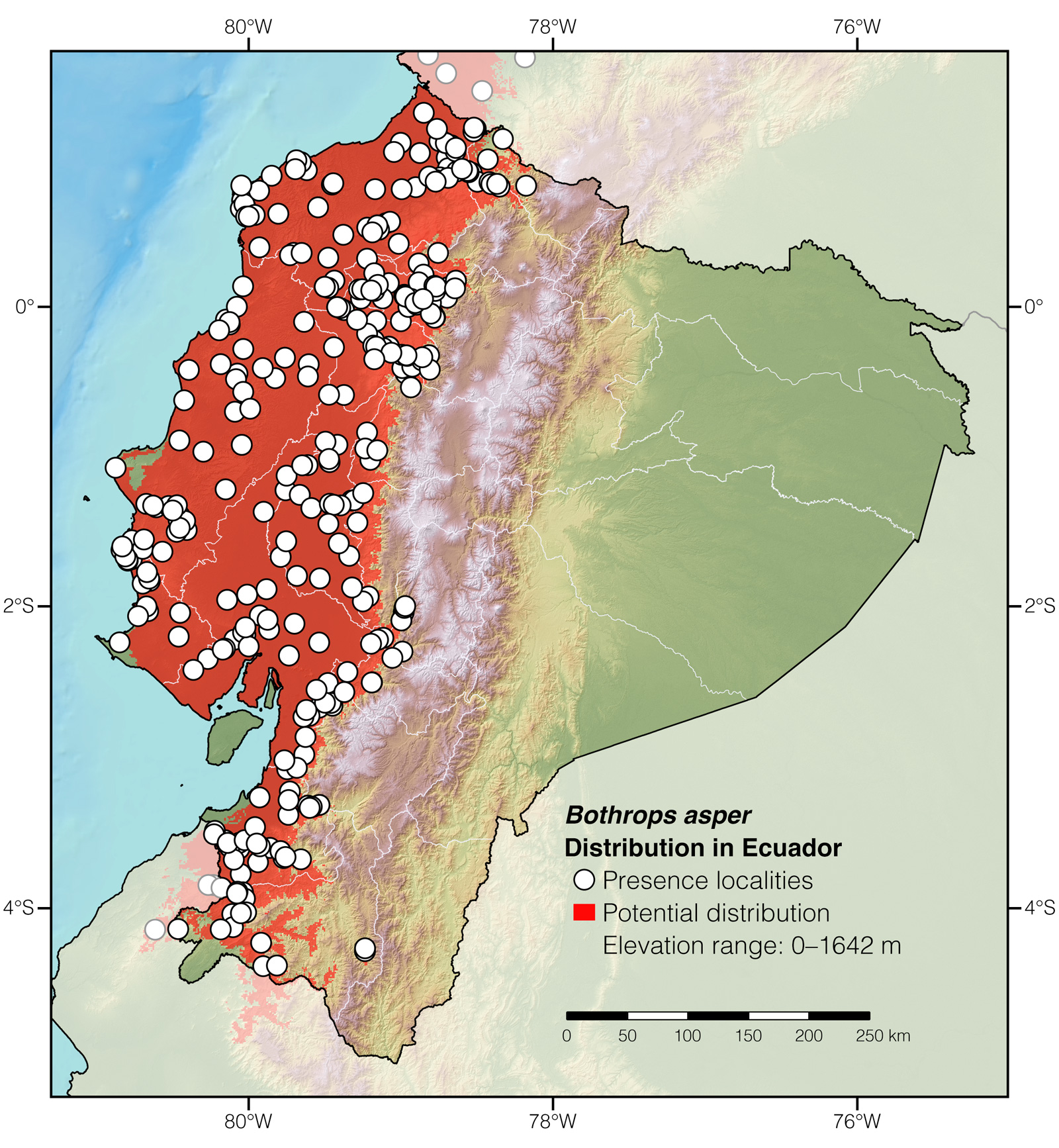
Figure 2: Distribution of Bothrops asper in Ecuador.
Etymology: The generic name Bothrops, which is derived from the Greek word bothros (meaning “pit”),66 refers to the heat-sensing pits between the eyes and nostrils. The specific epithet asper is a Latin word meaning “rough” or “harsh.” It probably refers to the skin on the dorsum of this species, which has a coarse texture.5
See it in the wild: Terciopelos can be located with ~10–30% certainty in forested or agricultural areas throughout western Ecuador. Some of the best localities to find Central American Lanceheads in the wild in Ecuador are: Bilsa Biological Reserve, Buenaventura Biological Reserve, Canandé Reserve, and Jama-Coaque Ecological Reserve. The snakes may be located by walking along trails at night.
FREQUENTLY ASKED QUESTIONS: |
Can you survive a viper bite? Yes, you can. With a timely treatment based on the appropriate antivenom, your chance of surviving is close to 100%. |
Can you eat a Fer-de-Lance? Yes, it is possible. The meat of the Fer-de-Lance is not toxic. It does, however, usually have a variety of parasitic worms.2,33 |
Do vipers follow humans? They don’t.1 In fact, in the case of the Terciopelo, there is evidence that the snakes actively avoid developed areas.12 They do, however, follow their prey, mostly rodents.12 Therefore, vipers such as the Fer-de-Lance are common where rat populations have exploded.5 |
How venomous is a Fer-de-Lance? With a lethal dose of LD50 1.9–11.2 mg/kg, the venom of the Fer-de-Lance is considered “extremely toxic.”35,36 In poorly managed or untreated human envenomations, the venom may cause permanent complications and disabilities in 6% of cases, and death in 5–7% of cases.47–49 |
Is the Fer-de-Lance the most venomous snake? No, it is not. Many snake species are more venomous. However, the Fer-de-Lance is responsible for the majority (44.5–100%)47,53,54 of snakebites throughout its range because snakes of this species are perfectly camouflaged, abundant in agricultural areas,11,49 have a high venom yield (up to 1,530 mg or 5–6 cc of venom per bite)49,55 and toxicity, and have an aggressive self-defense behavior.30,44 |
What does a Fer-de-Lance eat? The Fer-de-Lance has an opportunistic diet consisting largely (up to 69%) on mammals (mostly rodents, but also rabbits, skunks, opossums, and even porcupines), but also on amphibians, lizards, snakes, birds, invertebrates, fish, and carrion.2,26 |
Special thanks to Mahmood Sasa and Max Seldes for symbolically adopting the Fer-de-Lance and helping bring the Reptiles of Ecuador book project to life.
Click here to adopt a species.
Author: Alejandro ArteagaaAffiliation: Fundación Khamai, Reserva Arlequín, Ecoruta Paseo del Quinde km 56, Santa Rosa de Mindo, Pichincha 171202, Ecuador.
Photographers: Jose VieiraaAffiliation: Tropical Herping (TH), Quito, Ecuador.,bAffiliation: ExSitu, Quito, Ecuador. and Sebastián Di Doménico.
How to cite? Arteaga A (2020) Fer-de-Lance (Bothrops asper). In: Arteaga A, Bustamante L, Vieira J (Eds) Reptiles of Ecuador: Life in the middle of the world. Available from: www.reptilesofecuador.com. DOI: 10.47051/FEPX4083
Literature cited:
- Valencia JH, Garzón-Tello K, Barragán-Paladines ME (2016) Serpientes venenosas del Ecuador: sistemática, taxonomía, historial natural, conservación, envenenamiento y aspectos antropológicos. Fundación Herpetológica Gustavo Orcés, Quito, 653 pp.
- Sasa M, Wasko DK, Lamar WW (2009) Natural history of the Terciopelo Bothrops asper (Serpentes: Viperidae) in Costa Rica. Toxicon 54: 904–922. DOI: 10.1016/j.toxicon.2009.06.024
- Burger WL, Smith PW (1950) The coloration of the tail tip of young fer-de-lances: sexual dimorphism rather than adaptive coloration. Science 112: 431–433. DOI: 10.1126/science.112.2911.431
- Arteaga A, Pyron RA, Peñafiel N, Romero-Barreto P, Culebras J, Bustamante L, Yánez-Muñoz MH, Guayasamin JM (2016) Comparative phylogeography reveals cryptic diversity and repeated patterns of cladogenesis for amphibians and reptiles in northwestern Ecuador. PLoS ONE 11: e0151746. DOI: 10.1371/journal.pone.0151746
- Arteaga A, Bustamante L, Guayasamin JM (2013) The amphibians and reptiles of Mindo. Universidad Tecnológica Indoamérica, Quito, 257 pp.
- Wasko DK, Sasa M (2012) Food resources influence spatial ecology, habitat selection, and foraging behavior in an ambush-hunting snake (Viperidae: Bothrops asper): an experimental study. Zoology 115: 179–187. DOI: 10.1016/j.zool.2011.10.001
- Heimes P (2016) Snakes of Mexico. Chimaira, Frankfurt, 572 pp.
- Solórzano A (2004) Serpientes de Costa Rica. Distribución, taxonomía e historia natural. Universidad de Costa Rica, San José, 792 pp.
- Field notes, Reptiles of Ecuador book project.
- Freire-Lascano A, Kuch U (1994) A note on the geographical distribution of Bothrops asper (Garman, 1883) in Ecuador. Snake 26: 135–139.
- Hardy DL (1994) Bothrops asper (Viperidae) snakebite and field researchers in Middle America. Biotropica 26: 198–207. DOI: 10.2307/2388809
- Wasko DK, Sasa M (2009) Activity patterns of a Neotropical ambush predator: spatial ecology of the Fer-de-lance (Bothrops asper, Serpentes: Viperidae) in Costa Rica. Biotropica 41: 241–249. DOI: 10.1111/j.1744-7429.2008.00464.x
- Field notes of William Duellman.
- Acosta Vásconez AN (2014) Diversidad y composición de la comunidad de reptiles del Bosque Protector Puyango. BSc thesis, Universidad San Francisco de Quito, 157 pp.
- Medina-Barrios OD, Hernández-Cuadrado EE, Hernández Vélez D (2019) Termobiología de Bothrops asper (Garman, 1883) en Colombia: ensayos ecofisiológicos. Revista de Investigaciones Veterinarias del Perú 30: 61–73. DOI: 10.15381/rivep.v30i1.15673
- Vega-Coto J, Ramírez-Arce D, Baaijen W, Artavia-León A, Zúñiga A (2015) Bothrops asper. Arboreal behavior. Mesoamerican Herpetology 2: 199–201.
- Field notes of Brian Jimenez.
- Guyer C, Donnelly MA (2005) Amphibians and reptiles of La Selva, Costa Rica, and the Caribbean slope. University of California Press, Berkeley, 367 pp.
- McCranie JR (2011) The snakes of Honduras: systematics, distribution, and conservation. Society for the Study of Amphibians and Reptiles, Ithaca, 714 pp.
- Tryon BW (1985) Bothrops asper (Terciopelo). Caudal luring. Herpetological Review 16: 28.
- Kuch U, Boada C, García F, Freire A (2004) Bothrops asper (Terciopelo or Equis). Diet. Herpetological Review 35: 273–274.
- Leenders T (2019) Reptiles of Costa Rica: a field guide. Cornell University Press, Ithaca, 625 pp.
- Hertz A, Natera M, Lotzkat S, Sunyer J, Mora D (2009) Bothrops asper (Mapanare, Lancehead). Prey. Herpetological Review 40: 230.
- Parker HW (1926) The reptiles and batrachians of Gorgona Island, Colombia. Annals and Magazine of Natural History, London 17: 549–554. DOI: 10.1080/00222932608633442
- Boada C, Salazar-Valenzuela D, Freire-Lascano A, Kuch U (2005) The diet of Bothrops asper (Garman 1884) in the Pacific lowlands of Ecuador. Herpetozoa 18: 77–83.
- Farr WL, Lazcano D (2017) Distribution of Bothrops asper in Tamaulipas, Mexico and a review or prey items. Southwestern Naturalist 62: 77–84. DOI: 10.1894/0038-4909-62.1.77
- Cadena-Ortiz H, Barahona A, Bahamonde-Vinueza D, Brito J (2017) Anecdotal predation events of some snakes in Ecuador. Herpetozoa 30: 93–96.
- Logan CJ, Montero C (2009) Bothrops asper (Terciopelo). Scavenging behavior. Herpetological Review 40: 352.
- Ryan MJ, Blea NJ, Lantella IM, Kull MA (2010) Leptodactylus savagei (smoky jungle frog) antipredator defense. Herpetological Review 41: 337–338.
- Picado C (1931) Serpientes venenosas de Costa Rica. Sección Salud Pública Costa Rica, San José, 219 pp.
- Martins M, Marques OAV, Sazima I (2002) Ecological and phylogenetic correlates of feeding habits in Neotropical pitvipers of the genus Bothrops. In: Schuett GW, Höggren M, Douglas ME, Greene HW (Eds) Biology of the vipers. Eagle Mountain Publishing, Eagle Mountain, 307–328.
- Boinski S (2005) Use of a club by a wild white-faced capuchin (Cebus capucinus) to attack a venomous snake (Bothrops asper). American Journal of Primatology 14: 177–179. DOI: 10.1002/ajp.1350140208
- Quesada-Morúa R (2005) Hallazgos anatomo-histopatológicos en serpientes en cautiverio en Costa Rica. BSc thesis, Universidad Nacional, 81 pp.
- Moreno E, Bolaños R (1977) Hemogregarinas en serpientes de Costa Rica. Revista de Biología Tropical 25: 47–57.
- Terán MC, Lomonte B (2016) Actividad letal de seis venenos de serpientes de importancia médica en el Ecuador. Revista Ecuatoriana de Medicina y Ciencias Biológicas 37: 25–30. DOI: 10.26807/remcb.v37i2.4
- Kuch U, Mebs D, Gutiérrez JM, Freire A (1996) Biochemical and biological characterization of the Ecuadorian pitvipers (genera Bothriechis, Bothriopsis, Bothrops, and Lachesis). Toxicon 34: 714–717. DOI: 10.1016/0041-0101(96)00016-5
- Otero R, Gutiérrez JM, Núñez V, Robles A, Estrada R, Segura E, Toro MF, García ME, Díaz A, Ramírez EC, Gómez G, Castañeda J, Moreno ME (1996) Regional Group on Antivenom Therapy Research (REGATHER): a randomized double-blind clinical trial of two antivenoms in patients bitten by Bothrops atrox in Colombia. Transactions of the Royal Society of Tropical Medicine and Hygiene 90: 696–700. DOI: 10.1016/s0035-9203(96)90442-3
- Chaves F, Gutiérrez JM, Brenes F (1992) Pathological and biochemical changes induced in mice after intramuscular injection of venom from newborn specimens of the snake Bothrops asper (Terciopelo). Toxicon 30: 1099–1109. DOI: 10.1016/0041-0101(92)90055-a
- Gutiérrez JM, Chaves F, Bolaños RA (1980) Estudio comparativo de venenos de ejemplares recién nacidos y adultos de Bothrops asper. Revista de Biología Tropical 28: 341–351.
- Saldarriaga MM, Otero R, Núñez V, Toro MF, Díaz A, Gutiérrez JM (2003) Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 42: 405–411. DOI: 10.1016/s0041-0101(03)00171-5
- Mora-Obando D, Díaz C, Angulo Y, Gutiérrez JM, Lomonte B (2014) Role of enzymatic activity in muscle damage and cytotoxicity induced by Bothrops asper Asp49 phospholipase A2 myotoxins: are there additional effector mechanisms involved? PeerJ 2: e569. DOI: 10.7717/peerj.569
- Laines J, Segura Á, Villalta M, Herrera M, Vargas M, Alvarez G, Gutiérrez JM, León G (2014) Toxicity of Bothrops sp snake venoms from Ecuador and preclinical assessment of the neutralizing efficacy of a polyspecific antivenom from Costa Rica. Toxicon 88: 34–37. DOI: 10.1016/j.toxicon.2014.06.008
- Rucavado A, Soto M, Escalante T, Loría GD, Arni R, Gutiérrez JM (2005) Thrombocytopenia and platelet hypoaggregation induced by Bothrops asper snake venom. Toxins involved and their contribution to metalloproteinase-induced pulmonary hemorrhage. Thrombosis and Haemostasis 94: 123–131. DOI: 10.1160/TH05-02-0112
- Saborío P, González M, Cambronero M (1998) Snake bite accidents in children in Costa Rica: epidemiology and determination of risk factors in the development of abscess and necrosis. Toxicon 36: 359–366. DOI: 10.1016/s0041-0101(97)00076-7
- Otero‐Patiño R (2009) Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 54: 998–1011. DOI: 10.1016/j.toxicon.2009.07.001
- Otero R, Gutiérrez J, Mesa MB, Duque E, Rodrı́guez O, Arango JL, Gómez F, Toro A, Cano F, Rodrı́guez LM, Caro E, Martı́nez J, Cornejo W, Gómez LM, Uribe FL, Cárdenas S, Núñez V, Dı́az A (2002) Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 40: 1107–1114. DOI: 10.1016/s0041-0101(02)00104-6
- Otero R, Osorio RG, Valderrama R, Giraldo CA (1992) Efectos farmacológicos y enzimáticos de los venenos de serpientes de Antioquia y Chocó (Colombia). Toxicon 30: 611–620. DOI: 10.1016/0041-0101(92)90855-Y
- Russell FE, Walter FG, Bey TA, Fernandez MC (1997) Snakes and snakebite in Central America. Toxicon 35: 1469–1522. DOI: 10.1016/S0041-0101(96)00209-7
- Bolaños RA (1982) Las serpientes venenosas de Centro América y el problema del ofidismo. Aspectos zoológicos, epidemiológicos y biomédicos. Revista Costarricense de Ciencias Médicas 3: 165–184.
- Campbell JA, Lamar WW (2004) The venomous reptiles of the western hemisphere. Cornell University Press, Ithaca, 774 pp.
- Vargas-Baldares M (1978) Renal lesions in snakebite in Costa Rica. In: Rosenberg P (Ed) Toxins: animal, plant, and microbial. Pergamon Press, Oxford, 497.
- Otero R, Núñez V, Barona J, Díaz A, Saldarriaga M (2002) Características bioquímicas y capacidad neutralizante de cuatro antivenenos polivalentes frente a los efectos farmacológicos y enzimáticos del veneno de Bothrops asper y Porthidium nasutum de Antioquia y Chocó. Iatreia 15: 5–15.
- Sasa M, Vazquez S (2003) Snakebite envenomation in Costa Rica: a revision of incidence in the decade 1990–2000. Toxicon 41: 19–22. DOI: 10.1016/s0041-0101(02)00172-1
- Valencia JH, Barragán ME, Garzón K, Vargas A (2010) Reporte preliminar de mordedura de vipéridos en la provincia de Manabí, y notas sobre las áreas epidemiológicas con mayor concentración de serpientes venenosas. Gestión Ambiental 2: 9–13.
- Savage JM (2002) The amphibians and reptiles of Costa Rica, a herpetofauna between two continents, between two seas. The University of Chicago Press, Chicago, 934 pp.
- Morelet A (1871) Travels in Central America, including accounts on some regions unexplored since the Conquest. Leypoldt, Hold, and Williams, New York, 430 pp.
- Otero R, Núñez V, Osorio RG, Gutiérrez J, Giraldo CA, Posada LE (1995) Ability of six Latin American antivenoms to neutralize the venom of mapaná equis (Bothrops atrox) from Antioquia and Chocó (Colombia). Toxicon 33: 809–815. DOI: 10.1016/0041-0101(95)00009-b
- Alape-Girón A, Sanz L, Escolano J, Flores-Díaz M, Madrigal M, Sasa M, Calvete JJ (2008) Snake venomics of the lancehead pitviper Bothrops asper: geographic, individual, and ontogenetic variations. Journal of Proteome Research 7: 3556–3571. DOI: 10.1021/pr800332p
- Moreno E, Alape A, Sánchez M, Gutiérrez JM (1988) A new method for the detection of phospholipase A2 variants: identification of isozymes in the venoms of newborn and adult Bothrops asper (terciopelo) snakes. Toxicon 26: 363–371. DOI: 10.1016/0041-0101(88)90004-9
- Núñez V, Otero R, Barona J, Saldarriaga M, Osorio RG, Fonnegra R, Jiménez SL, Díaz A, Quintana JC (2004) Neutralization of the edema-forming, defibrinating and coagulant effects of Bothrops asper venom by extracts of plants used by healers in Colombia. Brazilian Journal of Medical and Biological Research 37: 969–977. DOI: 10.1590/s0100-879x2004000700005
- Avau B, Borra V, Vandekerckhove P, De Buck E (2016) The treatment of snake bites in a first aid setting: a systematic review. PLoS Neglected Tropical Diseases 10: e0005079. DOI: 10.1371/journal.pntd.0005079
- Solórzano A, Cerdas L (1989) Reproductive biology and distribution of the terciopelo, Bothrops asper Garman (Serpentes: Viperidae), in Costa Rica. Herpetologica 45: 444–450.
- Ripa D (2002) The bushmasters: morphology in evolution and behavior. Cape Fear Serpentarium, Wilmington, 349 pp.
- Carrillo E, Aldás A, Altamirano M, Ayala F, Cisneros-Heredia DF, Endara A, Márquez C, Morales M, Nogales F, Salvador P, Torres ML, Valencia J, Villamarín F, Yánez-Muñoz M, Zárate P (2005) Lista roja de los reptiles del Ecuador. Fundación Novum Millenium, Quito, 46 pp.
- Zipkin EF, DiRenzo GD, Ray JM, Rossman S, Lips KR (2020) Tropical snake diversity collapses after widespread amphibian loss. Science 367: 814–816. DOI: 10.1126/science.aay5733
- Brown RW (1956) Composition of scientific words. Smithsonian Books, Washington D.C., 882 pp.