Published November 6, 2021. Open access. | Purchase book ❯ |
Aquatic Coralsnake (Micrurus surinamensis)
Reptiles of Ecuador | Serpentes | Elapidae | Micrurus | Micrurus surinamensis
English common names: Aquatic Coralsnake, Amazonian Aquatic Coralsnake.
Spanish common names: Coral acuática, coral de agua.
Recognition: ♂♂ 126.2 cmMaximum distance from the snout to the tip of the tail. Snout–vent length=111.5 cm. ♀♀ 135 cmMaximum distance from the snout to the tip of the tail..1,2 In Ecuador, the majority of true coralsnakes can be distinguished from most, but not all, false coral snakes by having brightly colored rings that encircle the body, small eyes that are about the same size as the post-ocular scales, and no loreal scale.3,4 In the Amazon rainforest of Ecuador, Micrurus surinamensis is unmistakable due to its robust body, black rings arranged in triads, and red head cap with each scale outlined in black (Fig. 1).4 It is also unique among coral snakes in having the eyes and nostrils oriented dorsally, an adaptation for aquatic living.1 The presence of complete black triads separates this species from the Aesculapian False-Coralsnake (Erythrolamprus aesculapii), which, in Ecuador, has black rings arranged in dyads.5

Figure 1: Individuals of Micrurus surinamensis from Río Bigai Reserve () and from an unknown locality (), Orellana province, Ecuador.
Natural history: Micrurus surinamensis is an uncommon snake that inhabits pristine to heavily disturbed rainforests, which may be terra-firme or seasonally flooded,4 but also ventures into clearings, rural gardens, and houses near the forest border.6,7 Aquatic Coralsnakes are active at night, especially between sunset and midnight,3,8 but occasionally also during the day.3,7 They are semi-aquatic, which means individuals are usually, but not exclusively, found in or along slow-moving rivers, streams, swamps, ponds (including artificial tilapia ponds),3 and lagoons.3,9–11 They also forage on mud, leaf-litter, and even on vegetation.4,6 Juveniles have been seen coiled on vines and shrubs 1.35–1.38 m above the ground.12,13 These snakes actively forage above and below the water surface in search of prey. Their diet primarily includes swamp eels (Synbranchus marmoratus),4,14 knifefish (Gymnotus carapo4,15,14 and Sternopygus macrurus16), armored catfish (Callichthys callichthys),4,8,11 and other species of bony fishes,4,11 but also lizards7 and snakes (Atractus major6 and Erythrolamprus reginae17). An instance of predation was recorded in Brazil in which the snake slowly approached and seized the fish, bit the prey, and released it after envenomation. The ingestion process initiated after 10 minutes.8 Aquatic Coralsnakes rely on their warning coloration as a primary defense mechanism. Individuals are usually calm and try to flee when threatened.2 If disturbed, they engage in complex and seemingly erratic behavior: they hide the head beneath body coils, crawl spasmodically forward and then backward, flatten their body dorso-ventrally, and display their bright tails as a decoy.3–6 They are also capable of striking if provoked. Aquatic Coralsnakes are proteroglyphous and venomous (LD50The median lethal dose (LD50) is a measure of venom strength. It is the minium dosage of venom that will lead to the deaths of 50% of the tested population. 0.2–0.4 mg/kg).18 The venom appears to have evolved mainly to immobilize aquatic prey,18,19 since it is more lethal to fish than to mice, snakes, or amphisbaenians.18 The venom is mostly neurotoxic20–22 with little or not myotoxic (muscle breaking) activity,23 and the yield per bite is higher (up to 160 mg or 0.16 cc) than that of other Amazonian coralsnakes.2 In humans, it causes paresthesia (a burning or prickling sensation), blurred vision, muscle paralysis, difficulty breathing, and even death by respiratory arrest.2,24,25 Gravid females of M. surinamensis have been found to contain 5–12 eggs, and clutches of 8–13 eggs have been reported.3,2 The hatchlings measure 33–35 cm in total length.26
Conservation: Least Concern Believed to be safe from extinction given current circumstances..27–29 Micrurus surinamensis is included in this category because the species is widely distributed, occurs in major protected areas, has presumed stable populations, and is currently facing no major widespread extinction threats.27 The most important threat to the long-term survival of some populations is habitat destruction mostly due to mining, oil extraction, and the expansion of the agricultural frontier. Individuals of M. surinamensis also suffer from traffic mortality and direct killing at the hands of local people.3
Distribution: Micrurus surinamensis is widely distributed throughout the Amazon, occurring in Bolivia, Brazil, Colombia, Ecuador (Fig. 2), French Guiana, Guyana, Perú, and Suriname.
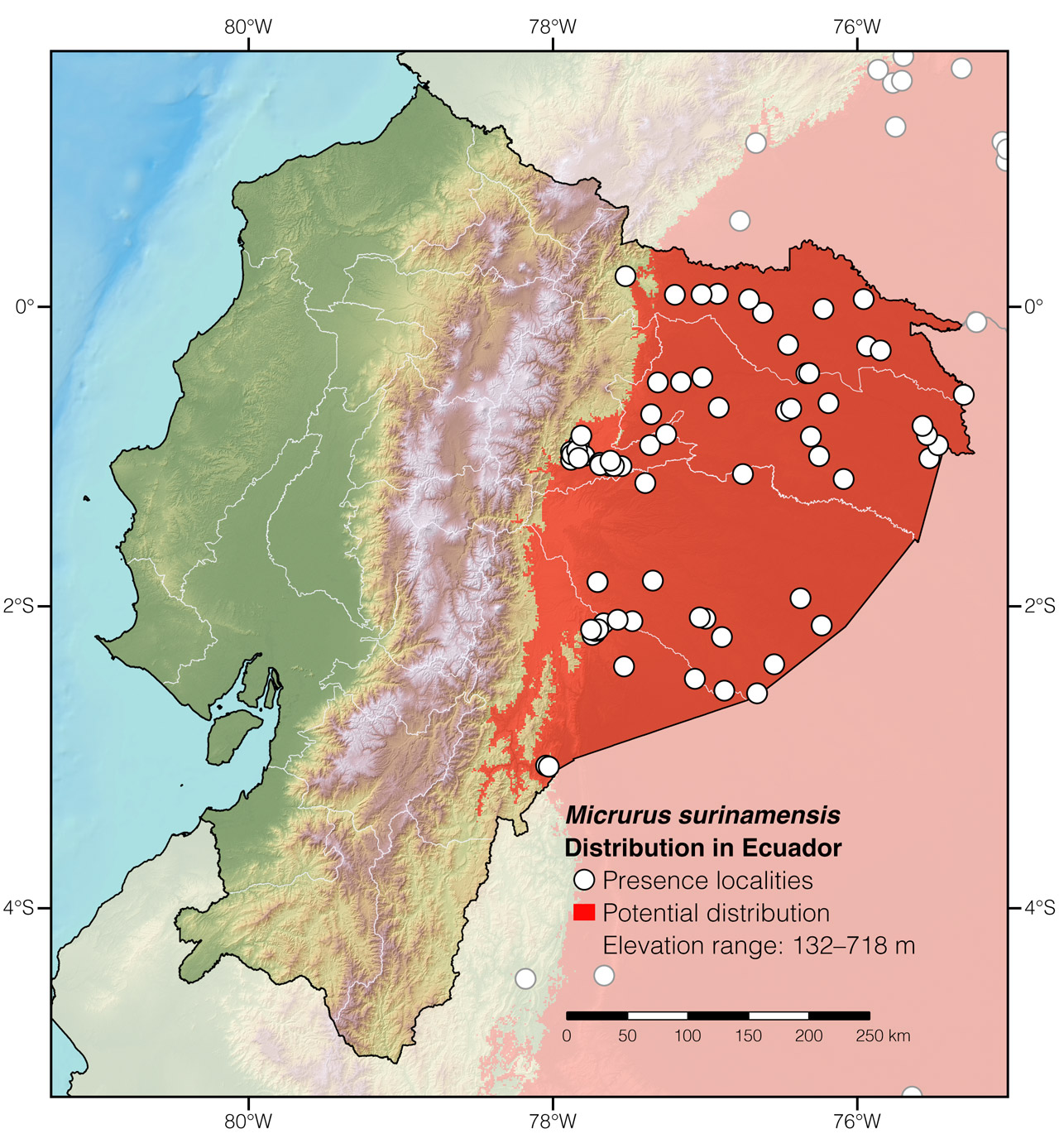
Figure 2: Distribution of Micrurus surinamensis in Ecuador. The type locality is Suriname. See Appendix 1 for a complete list of the presence localities included in the map.
Etymology: The name Micrurus is derived from the Greek words mikros (=small) and oura (=tail), referring to the short tail in members of this genus.4 The species epithet surinamensis refers to the type locality: Suriname.4
See it in the wild: Aquatic Coralsnakes are usually found no more than once every few weeks at any given locality. In Ecuador, the areas having the greatest number of recent observations are Sani Lodge, Yasuní Scientific Station, and Jatun Sacha Biological Reserve. It appears that the best way to find Aquatic Coralsnakes is to walk along forest streams and swamps right after sunset, especially after a warm and rainy day.
Special thanks to Pieter Staarink for symbolically adopting the Aquatic Coralsnake and helping bring the Reptiles of Ecuador book project to life.
Click here to adopt a species.
Author: Alejandro ArteagaaAffiliation: Fundación Khamai, Reserva Arlequín, Ecoruta Paseo del Quinde km 56, Santa Rosa de Mindo, Pichincha 171202, Ecuador.
Photographers: Jose VieirabAffiliation: Tropical Herping (TH), Quito, Ecuador.,cAffiliation: ExSitu, Quito, Ecuador. and Amanda QuezadaaAffiliation: Fundación Khamai, Reserva Arlequín, Ecoruta Paseo del Quinde km 56, Santa Rosa de Mindo, Pichincha 171202, Ecuador.
How to cite? Arteaga A (2021) Aquatic Coralsnake (Micrurus surinamensis). In: Arteaga A, Bustamante L, Vieira J (Eds) Reptiles of Ecuador: Life in the middle of the world. Available from: www.reptilesofecuador.com. DOI: 10.47051/TZUI8784
Literature cited:
- Duellman WE (1978) The biology of an equatorial herpetofauna in Amazonian Ecuador. Publications of the Museum of Natural History, University of Kansas 65: 1–352.
- Silva Haad JJ (1994) Los Micrurus de la Amazonia Colombiana. Biología y toxicología experimental de sus venenos. Colombia Amazónica 7: 1–76.
- Valencia JH, Garzón-Tello K, Barragán-Paladines ME (2016) Serpientes venenosas del Ecuador: sistemática, taxonomía, historial natural, conservación, envenenamiento y aspectos antropológicos. Fundación Herpetológica Gustavo Orcés, Quito, 653 pp.
- Campbell JA, Lamar WW (2004) The venomous reptiles of the western hemisphere. Cornell University Press, Ithaca, 774 pp.
- Curcio FF, Scali S, Rodrigues MT (2015) Taxonomic status of Erythrolamprus bizona Jan (1863 (Serpentes, Xenodontinae): assembling a puzzle with many missing pieces. Herpetological Monographs 29: 40–64. DOI: 10.1655/HERPMONOGRAPHS-D-15-00002
- Field notes, Reptiles of Ecuador book project.
- Martins M, Oliveira ME (1998) Natural history of snakes in forests of the Manaus region, Central Amazonia, Brazil. Herpetological Natural History 6: 78–150.
- Morais DH, Ávila RW, Kawashita-Ribeiro RA, Carvalho MA (2011) Squamata, Elapidae, Micrurus surinamensis (Cuvier, 1817): new records and distribution map in the state of Mato Grosso, Brazil, with notes on diet and activity period. Check List 7: 350–351.
- Silva Haad JJ, Rodríguez RA (1985) Las serpientes Micrurus de la Amazonía Colombiana. Amazonía 85: 26–28.
- Duellman WE (2005) Cusco amazónico: the lives of amphibians and reptiles in an Amazonian rainforest. Cornell University Press, Ithaca, 433 pp.
- Roze JA (1996) Coral snakes of the Americas: biology, indentification, and venoms. Krieger Publishing Company, Malabar, 328 pp.
- Hartdegen RW, Aucone B (2001) Natural history notes: Micrurus surinamensis surinamensis (NCN): arboreality. Herpetological Review 32: 264.
- Bertão Dávila PE, Takahashi HS, Oshiro Barbosa MH (2014) Micrurus surinamensis (Aquatic Coralsnake): behavior. Herpetological Review 45: 340.
- Schmidt KP (1952) The Surinam coral snake Micrurus surinamensis. Fieldiana: Zoology 34: 25–34.
- Bean BA (1924) A curious fish trap. Copeia 1924: 57–58.
- Tavares-Pinheiro R, Souza Melo F, Martins Barbosa de Figueiredo VA, Ribeiro Sanches P, Costa Anaissi JS, Salviano Santana MM, Oliveira-Souza AE, dos Santos Reis T, Lima Rebelo G, Vasconcelos Melo FT, Costa-Campos CE (2021) “In living color”: predation by the coralsnake Micrurus surinamensis (Cuvier, 1816) (Serpentes: Elapidae) on a knifefish in the eastern Amazon, Brazil. Herpetology Notes 14: 755–758.
- Pinto RR, Gomes M, Carvalho Jr R (2011) Micrurus surinamensis (Aquatic Coralsnake): ophiophagy. Herpetological Review 42: 441.
- da Silva Jr NJ, Aird SD (2001) Prey specificity, comparative lethality and compositional differences of coral snake venoms. Comparative Biochemistry and Physiology Part C 128: 425–456. DOI: 10.1016/s1532-0456(00)00215-5
- Sanz L, de Freitas-Lima LN, Quesada-Bernat S, Graça-de-Souzab VK, Soares AM, Calderón LdA, Calvete JJ, Caldeira CAS (2019) Comparative venomics of Brazilian coral snakes: Micrurus frontalis, Micrurus spixii spixii, and Micrurus surinamensis. Toxicon 166: 39–45. DOI: 10.1016/j.toxicon.2019.05.001
- Olamendi-Portugal T, Batista CVF, Restano-Cassulini R, Pando V, Villa-Hernandez O, Zavaleta-Martínez-Vargas A, Salas-Arruz MC, Rodríguez de la Vega RC, Becerril B, Possani LD (2008) Proteomic analysis of the venom from the fish eating coral snake Micrurus surinamensis: novel toxins, their function and phylogeny. Proteomics 8: 1919–1932. DOI: 10.1002/pmic.200700668
- Terra AL, Moreira-Dill LS, Simoes-Silva R, Monteiro JR, Cavalcante WL, Gallacci M, Barros NB, Nicolete R, Teles CB, Medeiros PS, Zanchi FB, Zuliani JP, Calderon LA, Stabeli RG, Soares AM (2015) Biological characterization of the Amazon coral Micrurus spixii snake venom: Isolation of a new neurotoxic phospholipase A2. Toxicon 103: 1-11. DOI: 10.1016/j.toxicon.2015.06.011
- Cecchini AL, Marcussi S, Silveira LB, Borja-Oliveira CR, Rodrigues-Simioni L, Amara S, Stábeli RG, Giglio JR, Arantes EC, Soares AM (2005) Biological and enzymatic activities of Micrurus sp. (Coral) snake venoms. Comparative Biochemistry and Physiology Part C 140: 125–134. DOI: 10.1016/j.cbpb.2004.11.012
- da Rocha Oliveira F, Nogueira Noronha MdD, Lopez Lozano JL (2017) Biological and molecular properties of yellow venom of the Amazonian coral snake Micrurus surinamensis. Revista da Sociedade Brasileira de Medicina Tropical 50: 365–373. DOI: 10.1590/0037-8682-0408-2016
- Warrell DA (2004) Snakebites in Central and South America: epidemiology, clinical features, and clinical management. In: Campbell JA, Lamar WW (Eds) The Venomous reptiles of the Western Hemisphere. Cornell University Press, Ithaca, 709–761.
- de Oliveira Pardal PP, de Oliveira Pardal JS, da Costa Gadelha MA, da Silva Rodrigues L, Feitosa DT, da Costa Prudente AL, Fan HW (2010) Envenomation by Micrurus coral snakes in the Brazilian Amazon region: report of two cases. Revista do Instituto de Medicina Tropical de São Paulo 52: 333–337. DOI: 10.1590/S0036-46652010000600009
- Starace F (1998) Serpents et amphisbènes de Guyane Française. Ibis Rouge Editions, Guadeloupe, 450 pp.
- Ines Hladki A, Ramírez Pinilla M, Renjifo J, Urbina N, Nogueira C, Gonzales L, Hoogmoed M, Schargel W, Rivas G (2019) Micrurus surinamensis. The IUCN Red List of threatened species. Available from: www.iucnredlist.org. DOI: 10.2305/IUCN.UK.2019-3.RLTS.T44582094A44582103.en
- Reyes-Puig C (2015) Un método integrativo para evaluar el estado de conservación de las especies y su aplicación a los reptiles del Ecuador. MSc thesis, Pontificia Universidad Católica del Ecuador, 73 pp.
- Carrillo E, Aldás A, Altamirano M, Ayala F, Cisneros-Heredia DF, Endara A, Márquez C, Morales M, Nogales F, Salvador P, Torres ML, Valencia J, Villamarín F, Yánez-Muñoz M, Zárate P (2005) Lista roja de los reptiles del Ecuador. Fundación Novum Millenium, Quito, 46 pp.
Appendix 1: Locality data used to create the distribution map of Micrurus surinamensis in Ecuador (Fig. 2). Go to the section on symbols and abbreviations for a list of acronyms used.
| Country | Province | Locality | Source |
| Colombia | Caquetá | El Rochal | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | El Rubí | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Florencia, 6 km NW of | iNaturalist |
| Colombia | Caquetá | Morelia | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Navarco | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Tres Esquinas del Caguán | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Valparaíso | iNaturalist |
| Colombia | Caquetá | Vereda La Floresta | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Vereda La Viciosa | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Vereda Los Ángeles | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Vereda Pore | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Caquetá | Vereda San Juan | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Putumayo | Finca San Carlos | Díaz-Ricaurte & Fiorillo 2019 |
| Colombia | Putumayo | Simón Bolívar | iNaturalist |
| Ecuador | Morona Santiago | Amazonas | Valencia et al. 2016 |
| Ecuador | Morona Santiago | Centro Kusutka | Valencia et al. 2016 |
| Ecuador | Morona Santiago | Kiim | Valencia et al. 2016 |
| Ecuador | Morona Santiago | Kushapucus | MZUA.RE.0197 |
| Ecuador | Morona Santiago | Macuma | Valencia et al. 2016 |
| Ecuador | Morona Santiago | Paantim | Valencia et al. 2016 |
| Ecuador | Morona Santiago | Santiago de Tiwintza | MCZ 45782 |
| Ecuador | Morona Santiago | Shuin Mamus | iNaturalist |
| Ecuador | Morona Santiago | Taisha | Photo by Axel Marchelie |
| Ecuador | Morona Santiago | Tunants | Valencia et al. 2016 |
| Ecuador | Napo | Archidona | Online multimedia |
| Ecuador | Napo | Atacapi | iNaturalist |
| Ecuador | Napo | Chontapunta | Valencia et al. 2016 |
| Ecuador | Napo | Finca Fischer | TCWC 69458 |
| Ecuador | Napo | Jatun Sacha Biological Station | Vigle 2008 |
| Ecuador | Napo | Liana Lodge | iNaturalist |
| Ecuador | Napo | Misahuallí | KU 202957 |
| Ecuador | Napo | Muyuna | Valencia et al. 2016 |
| Ecuador | Napo | Puerto Mishuallí | iNaturalist |
| Ecuador | Napo | Rancho Johanna | TCWC 68725 |
| Ecuador | Napo | San Pedro | Valencia et al. 2016 |
| Ecuador | Napo | Sinchi Sacha | Photo by Ernesto Arbeláez |
| Ecuador | Napo | Suchipakari Lodge | This work |
| Ecuador | Napo | Tena | iNaturalist |
| Ecuador | Napo | Tena, 5 km SW of | Valencia et al. 2016 |
| Ecuador | Napo | Yachana Reserve | Whitworth & Beirne 2011 |
| Ecuador | Napo | Yuralpa | This work |
| Ecuador | Napo | Zoo el Arca | Photo by Diego Piñán |
| Ecuador | Orellana | Campo Repsol YPF | Valencia et al. 2016 |
| Ecuador | Orellana | Dayuma | Valencia et al. 2016 |
| Ecuador | Orellana | El Coca | MHNG 2249.017 |
| Ecuador | Orellana | El Coca, 15 km W of | iNaturalist |
| Ecuador | Orellana | Laguna de Jatuncocha | Valencia et al. 2016 |
| Ecuador | Orellana | Margen del Río Nashiño | Valencia et al. 2016 |
| Ecuador | Orellana | NPF | Photo by Neshat Shoghi |
| Ecuador | Orellana | Nuevo Rocafuerte | Valencia et al. 2016 |
| Ecuador | Orellana | Puerto Miranda | iNaturalist |
| Ecuador | Orellana | San José de Payamino | Maynard et al. 2016 |
| Ecuador | Orellana | Tiputini | USNM 232496 |
| Ecuador | Orellana | Tiputini Biodiversity Station | iNaturalist |
| Ecuador | Orellana | Vía Hollín–Loreto, km 77 | Valencia et al. 2016 |
| Ecuador | Orellana | Vía Pompeya Sur–Iro, km 86 | Valencia et al. 2016 |
| Ecuador | Orellana | Yasuní Scientific Station | This work |
| Ecuador | Pastaza | Andoas | Valencia et al. 2016 |
| Ecuador | Pastaza | Anga Cocha | Nogueira et al. 2019 |
| Ecuador | Pastaza | Balsaura | Ortega-Andrade 2010 |
| Ecuador | Pastaza | Boca del Río Capahuari | Valencia et al. 2016 |
| Ecuador | Pastaza | Juyuintza | Ortega-Andrade 2010 |
| Ecuador | Pastaza | Killu Allpa | Nogueira et al. 2019 |
| Ecuador | Pastaza | Montalvo | Valencia et al. 2016 |
| Ecuador | Pastaza | Río Copataza | Valencia et al. 2016 |
| Ecuador | Pastaza | Río Corrientes | USNM 232497 |
| Ecuador | Pastaza | Río Pastaza | Valencia et al. 2016 |
| Ecuador | Pastaza | Río Rutuno | USNM 232499 |
| Ecuador | Pastaza | Río Tzapino | Campbell & Lamar 2004 |
| Ecuador | Pastaza | Shiripuno Lodge | Online multimedia |
| Ecuador | Pastaza | Uyuimi | Online multimedia |
| Ecuador | Pastaza | Uyuimi | Photo by Darwin Núñez |
| Ecuador | Sucumbíos | Campo Libertador | Valencia et al. 2016 |
| Ecuador | Sucumbíos | Campo Unita | Valencia et al. 2016 |
| Ecuador | Sucumbíos | Dureno | Yánez-Muñoz & Chimbo 2007 |
| Ecuador | Sucumbíos | Garzacocha | Yánez-Muñóz & Venegas 2008 |
| Ecuador | Sucumbíos | Lagartococha | USNM 232495 |
| Ecuador | Sucumbíos | Lago Agrio | Valencia et al. 2016 |
| Ecuador | Sucumbíos | Playas del Cuyabeno | Valencia et al. 2016 |
| Ecuador | Sucumbíos | Reserva de Producción Faunística Cuyabeno | FMNH 2008 |
| Ecuador | Sucumbíos | Río Aguarico | Nogueira et al. 2019 |
| Ecuador | Sucumbíos | San Pablo de Kantesiya | Valencia et al. 2016 |
| Ecuador | Sucumbíos | San Pedro de los Cofanes | iNaturalist |
| Ecuador | Sucumbíos | Sani Lodge | This work |
| Ecuador | Sucumbíos | Santa Cecilia | Duellman 1978 |
| Ecuador | Sucumbíos | Sevilla | iNaturalist |
| Perú | Amazonas | Aguaruna Village | MVZ 163331 |
| Perú | Amazonas | Caterpiza | USNM 566631 |
| Perú | Amazonas | Huampami | USNM 316654 |
| Perú | Amazonas | Teniente Pinglo | USNM 566634 |
| Perú | Loreto | Aguas Negras | FMNH 2008 |
| Perú | Loreto | Puerto Nuevo de Parinari, 7 km S of | iNaturalist |
| Perú | Loreto | Reserva Nacional Pacaya Samiria | iNaturalist |
| Perú | Loreto | Zona de Reserva Güepi | iNaturalist |